Abstract
Background: Many patients with Acute Myeloid Leukemia (AML) diagnosed after 60 years of age are not considered suitable for intensive remission induction chemotherapy, either due to co-morbidities or frailty associated with advanced age. Despite treatment with either a hypomethylating agent or low-dose cytarabine arabinoside (LDAC), survival is usually poor, with 1-year survival after LDAC of 24-37% in NCRI AML16 and historical arms of LI-1. Combination therapy with additional agents represents an attractive option, and has the potential to improve patient outcomes without substantially increasing toxicity. Lenalidomide (Revlimid TM) is an immunomodulatory drug, used to treat myeloma, and some cases of myelodysplastic syndrome, and has potent antineoplastic, anti-angiogenic, anti-inflammatory and pro-erythropoietic properties. Early-phase trials of lenalidomide in AML have demonstrated clinical activity with acceptable toxicity.
Aim: To assess the efficacy and tolerability of LDAC+lenalidomide versus LDAC alone in patients aged 60+ unsuitable for intensive therapy. The aim was to double 2-year survival from 11% to 22% (HR 0.69).
Methods: LI-1 was an international multicentre, multi-arm, randomised phase II trial developed to study the efficacy and tolerability of novel non-intensive therapies in AML using a "pick a winner" design. LDAC was given at 20mg BD SC on days 1-10 of each course. Lenalidomide was administered orally once daily in a flat 10mg dose for 21 days, where day 1 is day 1 of LDAC with courses occurring at 5-week intervals for courses 1-4. Patients considered to be benefitting after 4 courses, i.e. in remission or stable disease, could continue to receive treatment until disease progression, either with LDAC+lenalidomide at 6-week intervals, or lenalidomide only at 4-week intervals if patient had experienced significant toxicity. Toxicities were recorded using CTCAEv3. Primary objectives included overall survival (OS), complete remission (CR + CRi) achievement, reasons for failure, duration of response (CR/CRi), relapse rates and deaths in first CR. Secondary objectives included toxicity, supportive care requirements, and Quality of Life assessments (measured using EORTC QLQ-30, EQ5D and HADS tools).
Results: Between Jan-17 and Jun-19, 202 patients from Denmark (8%), New Zealand (16%) and the UK (76%) were randomised. Median age was 78 years (range 62-89). Overall, 58% were male; 76% de novo AML, 20% secondary AML, and 4% high risk MDS; 1% favourable, 66% intermediate, 19% adverse and 14% unknown cytogenetics; WHO performance status 0 for 15%, 1 for 58%, 2 for 22% and 3 for 5%. Median of 2 courses (range 0-24; mean 3.28) was delivered in LDAC arm and 1 course in LDAC+lenalidomide arm (range 0-25; mean 3.48).
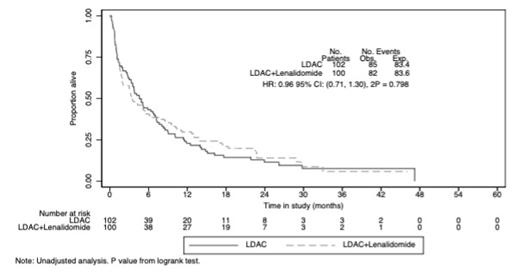
Overall response (CR/CRi) was achieved in 40/202 patients (20%), (LDAC+lenalidomide 25%, LDAC 14%, OR 0.45 (0.22, 0.93), P=0.031). 30-day mortality was not significantly increased (19% in both arms); and 2-year OS showed no significant difference (14% vs 11.5%, HR 0.94 (0.69, 1.29, P=0.719). Median OS was 3.5 vs 4.6 months; HR 0.96 (0.71, 1.30), P=0.80. 1-year OS for patients that didn't enter CR/CRi was 6.8% for LDAC+lenalidomide vs 16.9% for LDAC (P=0.028). Cause of death for the majority of patients was resistant/recurrent disease: 45(53%) vs 47(58%). Most adverse events (AEs) were grade 1/2 in both arms. During cycle 1, there were 78 vs 51 grade 3/4 AEs in the LDAC+lenalidomide and LDAC arms, respectively (P=0.02). This included 5 thrombotic events in the LDAC+lenalidomide arm (4 grade 3 and 1 grade 4) and none in the LDAC arm. In course 1, supportive care requirements were higher in terms of both days of antibiotics (7 vs 3; p=0.001) and hospitalisation days (11 vs 6.5; p=0.005) for the LDAC+lenalidomide arm. There was no difference in transfusion requirements.
Conclusions: Despite improving the CR/CRi rate, the combination of lenalidomide+LDAC did not improve OS, relapse-free survival or time in remission in elderly patients with AML. The addition of lenalidomide to LDAC resulted in increased toxicity, including episodes of thrombosis, as well as increased supportive care requirements. Alternative strategies to improve survival for elderly patients with AML remain a significant clinical need.
Acknowledgements: We are grateful to Blood Cancer UK for funding the trial and Celgene for providing drug and additional support for this IIS.
Fig 1: OS All patients
Copland: Astellas: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Incyte: Honoraria, Research Funding, Speakers Bureau; Jazz: Honoraria, Speakers Bureau; Cyclacel Ltd: Research Funding. Russell: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz: Research Funding, Speakers Bureau.
Off label use of lenalidomide in combination with low dose cytarabine will be discussed in the setting of elderly unfit AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal